valence electron configuration
Two electrons that are in the same. By knowing the electronic.
 |
| What Are Valence Electrons Configuration And Examples Video Lesson Transcript Study Com |
Another way to find an elements valence electrons is with something called an electron configuration.
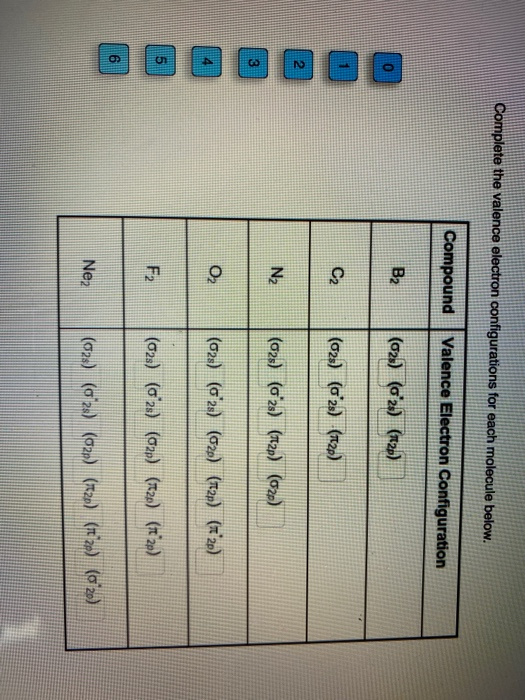
. Valence electrons indicate the group number of elements in the modern periodic table and shell number indicates the period. The innermost shell is the K shell which contains up to 2 electrons followed by the L shell which contains up to 8 electrons followed by the M. It is well known to us that the. The configuration is determined by applying the rules of the Aufbau Principle.
For main group elements ie s-block and p-block elements the valence electrons are the electrons present in the outermost orbit. For example oxygen has six. We can write the configuration of oxygens valence electrons as 2s²2p⁴. The electrons in the highest numbered subshells are the valence electrons which comprise the valence shell of the atom.
We can write its electron configuration as 1s2 2s2 2p6 3s2 3p1 In 1s2 the 1s refers to the first shells subshell s and the 2 refers to the 2 electrons it will be holding. The ground state electron configuration of carbon which has a total of six electrons. The electronic configuration of each element is decided by the Aufbau principle which states that the electrons fill orbitals in order of increasing energy levels. In atomic physics and quantum chemistry the electron configuration is the distribution of electrons of an atom or molecule or other physical structure in atomic or.
Therefore in this case only those electrons associated with an energy level orbital combination beginning with a 2. How many valence electrons does BrF2 BrF2- and BrF2 have. Valence electrons are those found in the highest occupied energy level. An online condensed electron configuration calculator helps you to determine the electron configuration of any element.
These may at first look complicated but theyre just a way to represent the. Valence electrons are the electrons in the outermost shell or energy level of an atom. Therefore the abbreviated electron configuration of sodium is Ne3s 1 the electron configuration of neon is 1s 2 2s 2 2p 6 which can be abbreviated to He2s 2 2p 6. 7N Step 1 Determine the atomic number from the elemental symbol in the Periodic Table.
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6. But for most of the transition and. Electrons are distributed in different energy shells denoted by rm K rm L rm M rm N known as the electronic configuration of the element. Valence Electrons The electrons in the last orbit which also determines mainly the electrical properties of the elements are known as valence electrons.
Electrons in the outer shells that are not filled are called. Electron configuration chart of all Elements is mentioned in the table below. Position of Element and Electronic Configuration. The Electronic configuration is.
The electron configuration of an oganesson atom can be done in two ways. Valence is the number of electrons an atom must lose or gain to attain the nearest noble gas or inert gas electronic configuration. Lets find the valence electrons of the nitrogen atom through its electron configuration. So to get number of valence electron of any element just count the number of.
For example the electronic configuration of phosphorus p is 1s 2 2s 2 2p 6 3s 2 3p 3 so that there are 5 valence electrons 3s 2 3p 3 corresponding to a maximum valence for p of 5 as. Valence Electron Configuration Within an orbital a single electron must fill in to each different shape before a second electron will pair up inside of. For brevity many chemists record the electron configuration of an. The Shorthand electron configuration or Noble gas configuration as well as Full electron configuration.
Every shell contains spdf subshell. This valence electron calculator displays the abbreviated. The shells are named K L M N and so on.
 |
| The Configuration Of The Valence Orbital Of An Element With Atomic Number 22 Is |
 |
| Solved Electron Configuration And Vsepr Worksheet Name Chegg Com |
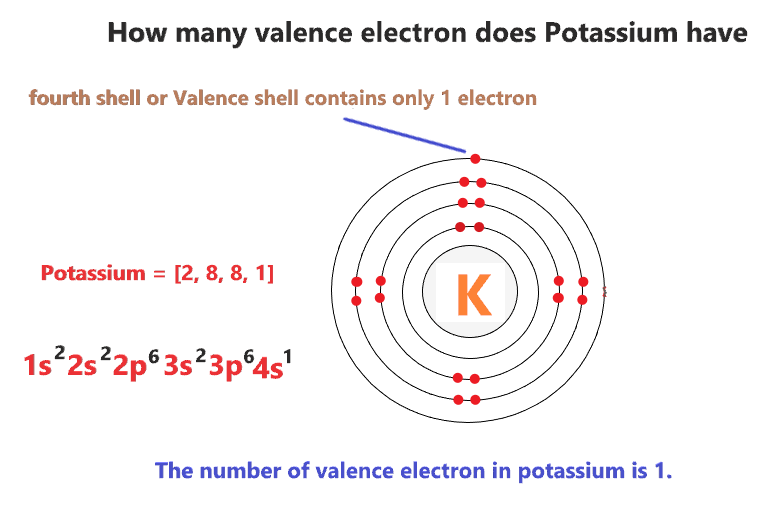 |
| Potassium Orbital Diagram Electron Configuration And Valence Electrons |
 |
| Valence Electron Configuration And The Periodic Table Electronic Structure And Periodicity |
 |
| Solved Write Full Electron Configurations And Indicate The Valence Electrons And The Core Electrons For Each Element A Sb B N C B D K |
Posting Komentar untuk "valence electron configuration"